GcMAF
Background
GcMAF (Gc Protein-derived Macrophage Activating Factor) occurs naturally in the human body and activates macrophages to destroy cancer cells and foreign invaders such as bacteria and viruses. Serious illnesses like cancer, HIV and viral hepatitis are destroyed by GcMAF.



GcMAF neutralizes our immune system to defend itself. This allows the disease to progress uncontrolled. We use a small sample of healthy human serum to produce large amounts of new second-generation GcMAF in our specialized sterile laboratory, the Cell Processing Center (CPC). This highly active second-generation Gc-MAF is injected intramuscularly (IM) or subcutaneously (SC) into the patient, usually twice and in some cases 3 times a week. Over a matter of weeks and months, the immune system is strengthened through the activation of macrophages and begins to eradicate cancer cells, viruses and bacteria. In addition to GcMAF injections, another form of GcMAF manufactured from high-quality colostrum can be administered orally, in the gut and sublingually to activate macrophages in the lymphoid tissue.
General Goals of GcMAF Therapy
- Improve well-being and quality of life (QOL)
- Return the patient to good health so that they are able to participate in regular lifestyle activities
- Achieve long-term survival
- Enhance the effects of other therapies
- Repair the immune system
- Increase the number of monocytes (macrophages) and activate them to destroy cancer cells, viruses, bacteria and other pathogens in the body
- Increase the rate of maturation of dendritic cells (DCs)
GcMAF Therapy Overview
- One course of High-Dose GcMAF is usually 48 doses for 6 months (2 times weekly administration).
- For advanced conditions, High-Dose GcMAF may be administered 3 times a week.
- Additional courses may be required depending on the stage of the disease and other factors specific to each patient.
- Treatment should be continued at a high dose as long as the disease is present to destroy cancer cells, viruses, bacteria and other pathogens in the body.
- For serious diseases, we recommend a combination of GcMAF injection and daily Colostrum MAF by oral and sublingual administration for the most effective treatment.
- Long-term maintenance doses of High-Dose GcMAF may be important to reduce recurrence after evidence of disease eradication.
- Oral Colostrum MAF may be a convenient long-term option to maintain health.
Other Important Points
- Activating macrophages with High-Dose GcMAF is an important part of any treatment program which can be used alone or in combination with most other therapies.
- GcMAF works especially well in synergy with targeted therapies which don’t harm the immune system. Examples of targeted therapies include hormone therapies, monoclonal antibody drugs, small-molecule drugs, signal transduction inhibitors (HER2 inhibitors, BRAF inhibitors, EGFR inhibitors), angiogenesis inhibitors, immunotherapy drugs (such as drugs that target CTLA-4 protein).
- Second-generation GcMAF has the advantage of having no side effects so treatment should be continued as long as necessary while the disease is present. This is a significant advantage over many conventional therapies which have cumulative toxicity that limits their use.
- GcMAF never stops working and will continue to activate macrophages while treatment is continued, either by GcMAF injections and/or oral administration of Colostrum GcMAF.
Second-Generation GcMAF
Second-generation Gc-MAF is produced using a new patented process which was developed here in Japan by Saisei Mirai in collaboration with researchers from the University of Tokushima who have been studying GcMAF for over 20 years. Our GcMAF is made in our sterile cell processing facility using this new and improved 2nd generation method which is 10-15 times more concentrated and is more active and stable than other GcMAF currently available. More importantly, this much higher concentration GcMAF has been clinically demonstrated to be largely free of any side effects in the great majority of patients and is much more stable as it is resistant to oxidation. Only low grade fever or eczema have been observed in about 1 out of 100 patients using GcMAF but these were short-term effects.
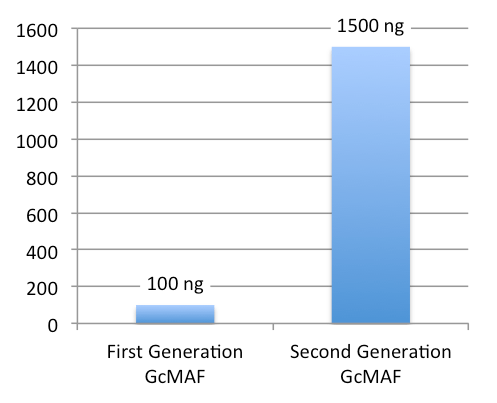
First-Generation GcMAF vs Saisei Mirai Second-Generation GcMAF Concentration
- 0.5 ml of High-Dose GcMAF is approximately 1500 ng GcMAF
- Gc-MAF is a natural immunotherapy product. Variation in GcMAF concentration is due to normal variation between serum samples. In the same way that lymphocytes or natural killer cells vary in number between people at any given time, so will the amount of GcMAF that can be produced from serum.
- Our GcMAF is produced under aseptic conditions in a specialized facility and sterile filtration is used in the production of all product.
- Our new 2nd generation Gc-MAF has been safely used in hundreds of patients in our clinic in Japan, since April 2011. Treatment in our clinic has been by Intramuscular (IM), Subcutaneous (SC) and Intramural (IT) injection.
How is Second-Generation GcMAF Made?
Second-generation GcMAF is manufactured in our own sterile Saisei Mirai Cell Processing Center (CPC) from healthy human serum which is carefully screened and the final product sterile filtered to ensure safety. See the GcMAF Tests below for more details.
https://youtu.be/X9sU4-gvkBQ
https://youtu.be/l_HF8OSlLMk
Diagram showing the steps of the GcMAF preparation process

Photo courtesy, University of Tokushima
Macrophage phagocytic activity of second-generation GcMAF. The purple color are macrophages activated by GcMAF phagocytizing (ingesting) opsonized red blood cells which are clear in color.
What are Macrophages? Macrophages (Greek: big eaters) are cells produced by the differentiation of monocytes, a type of white blood cell, in tissues. Macrophages function in both non-specific defense (innate immunity) as well as help initiate specific defense mechanisms (adaptive immunity) of vertebrate animals. Their role is to phagocytose (engulf and then digest) cellular debris and pathogens, either as stationary or as mobile cells. They also stimulate lymphocytes and other immune cells to respond to pathogens. They are specialized phagocytic cells that attack foreign substances, infectious microbes and cancer cells through destruction and ingestion. M1 macrophages – cancer suppressors
New Research papers published by Saisei Mirai
Clinical application of INUI-X 1500-Protein Introduction to INUI-X 1500-Protein contains GcMaf 2018
Steps of a Macrophage Ingesting a Pathogen 
- Ingestion through phagocytosis, a phagosome is formed
- The fusion of lysosomes with the phagosome creates a phagolysosome; the pathogen is broken down by enzymes
- Waste material is expelled or assimilated (the latter not pictured)
Parts:
- Pathogens
- Phagosome
- Lysosomes
- Waste material
- Cytoplasm
- Cell membrane
Vitamin D-Binding Protein Vitamin D binding protein is also known as Gc Protein. It is produced in our body, mainly in the liver, especially when we are exposed to the sun. This binding protein binds to 25 (OH) vitamin D in our body for transport and storage. There are different forms of Vitamin D BP, the most dominant being non-glycosylated 656 Da proteins. Vitamin DBP is the most important scavenger of extracellular G-actin, important in liver disease. Vitamin DBP activates macrophages through GaINAc- modified Gc Protein. Vitamin DBP has virtually no impact on the distribution, uptake, activation profile, or biological potency of the hormone vitamin D in our body, so too much is unlikely to be a problem. Vitamin D-binding protein is the basic macrophage activating factor in our body. Factors that Influence Vitamin DBP Levels Liver disease decreases levels of Vitamin DBP (Gc Protein). Chronic liver disease will decrease levels less than acute liver failure. Trauma and surgery will decrease Vitamin DBP. Septic infections will consume Vitamin DBP faster than production can be increased. Normal Vitamin DBP (Gc Protein) levels in serum are 350-500 mg/l. Levels of Gc Protein less than 80 mg/l yield positive and negative mortality predictive values of 85% and 43% respectively. Survivors had levels greater than 102 mg/l. What is the Macrophage Activation Factor? Macrophage activation factor (MAF) are glycoproteins that increase macrophage activity and transform them into natural killer (NK) cells. Vitamin DBP (Gc Protein) is the primary MAF. The glycosylated Gc Protein is the best MAF.
Diseases for GcMAF Therapy
- Gc-MAF macrophage activation therapy is useful in the treatment of many diseases, such as cancer, HIV AIDS, Hepatitis B virus (HBV), Hepatitis C virus (HCV), Herpes Simplex virus (HSV), Tuberculosis, Pneumonia infection, Epstein-Barr virus (EBV), cystitis/urinary tract infection (UTI), Endometriosis, Selective IgA deficiency disorder and influenza virus.
- In healthy individuals the immune system may be able to overcome many kinds of diseases, however people with a compromised immune system will benefit from GcMAF therapy.
- In the great majority of people there are no side-effects with our 2nd generation Gc-MAF therapy, or side-effects are very minor and extremely rare. Low grade fever and eczema has been observed in about 1 out of 100 patients using GcMAF but these were short-term effects.
- Treatment in our clinic has been by Intramuscular (IM), Subcutaneous (SC) and Intramural (IT) injection.
In Combination With Other Treatments GcMAF can be safely used with a wide variety of other standard treatments and drugs to improve their effect. We refer to this as integrative medicine.
- A combination with anti-cancer drugs and radiation therapy (radiotherapy) is possible. For maximum effect and benefit from GcMAF, administer a few days apart from chemotherapy. Radiation therapy does not have significant effects on Gc-MAF, so both can be used together at any time. In our clinical experience we have observed significant cancer killing effects from GcMAF combined with palliative radiotherapy in patients who underwent substantial prior chemotherapy treatment. See our Case Reports for more details on this multimodality integrative treatment.
- Studies show that GcMAF has anti-angiogenic activity in addition to tumor killing activity through the activation of macrophages.
- GcMAF can be combined with Sonodynamic Therapy (SDT), Photodynamic Therapy (PDT) or both (Sonophotodynamic Therapy, SPDT), Maitake Extract, Coley Vaccine (Coley Fluid), high-dose IV Vitamin C, low dose Naltrexone (LDN), Alpha-Lipoic Acid, hyperthermia therapy, immunotherapies and cancer vaccines (such as autologous cancer vaccine).
- GcMAF should be used in combination with at least 5,000 IU vitamin D3 daily. Blood levels of vitamin D are often low in many diseases such as cancer, HIV AIDS, etc. Normal vitamin D levels are required for GcMAF to work fully. Have your blood 25 hydroxy-vitamin D and calcium levels tested. If blood calcium levels become elevated, vitamin D3 doses may need to be reduced to achieve an optimal balance.
Combinations to Avoid Gc-MAF can be safely used with a wide variety of drugs and other treatments. However, we recommend:
- Minimal use of steroids is desirable because of their immune suppressing effect, however steroids may be safely used with GcMAF if necessary and prescribed by your physician.
- Radiation therapy is preferred over chemotherapy whenever possible.
Treatment
- Treatment is by intramuscular (IM) or subcutaneous (SC) injection of the GcMAF macrophage activating factor, 1-2 times per week (or as prescribed by the treating physician). See the dosing recommendations below.
- Treatment in our clinic has also been by intramural (IT) injection although IM and SC injections are by far the most common method of administration.
- Good aseptic handling with ethanol is required when using the vials.
Dosing Recommendations for Second-generation GcMAF
- Dosage and frequency of Gc-MAF administration are at the discretion of the treating physician.
- No upper limit has been established for second-generation GcMAF.
Cancer, HIV AIDS, Hepatitis, Tuberculosis For patients with cancer, HIV AIDS, hepatitis and tuberculosis, we suggest 1500 ng High-Dose GcMAF once or twice per week.
- For maximum effect we recommend 0.5 ml twice weekly.
- One course of High-Dose GcMAF is usually 48 doses for 6 months. Additional courses may be required depending on the stage of the disease and the severity of the symptoms.
Macrophage activation is always required for an effective functioning of the immune system. Gc-MAF therapy should continue as long as the disease is present and for a period afterwards to reduce the chance of recurrence. Chronic Fatigue Syndrome (CFS)/Myalgic Encephalomyelitis (ME) In the Chronic Fatigue Syndrome (CFS) and Myalgic Encephalomyelitis (ME), a lower dosage of 100 ng Low-Dose GcMAF once per week is commonly recommended. With second-generation GcMAF, we recommend using High-Dose GcMAF for a more effective treatment. Recommended dose – 0.25ml High-Dose GcMAF twice a week by intramuscular or subcutaneous injection.
- Improvements in symptoms should be observed within 2 months.
- The minimum treatment course is 6 months but each patient is different and additional courses may be required based on positive progress.
- Patients may need long-term maintenance doses of GcMAF therapy to stay well and symptom-free until their immune system is fully recovered to cope with any challenge.
- If the patient already uses 100 ng Low Dose GcMAF and the effects from the treatment are not pronounced, we recommend switching to second-generation High-Dose GcMAF.
- In our clinic in Japan, all of our patients, regardless of the disease, use second-generation High-Dose GcMAF.
* Note that these dosage recommendations apply only to Saisei Mirai second-generation GcMAF. Autism Spectrum Disorders (ASD) Recommended dose – 0.25ml High-Dose GcMAF twice weekly by intramuscular or subcutaneous injection. *
- Some improvements in symptoms should be observed within 2 months.
- The minimum treatment course is 6 months but each individual patient is different and additional courses may be required based on positive progress.
- Patients may need long-term maintenance doses of GcMAF therapy to stay well and symptom-free until their immune system is fully recovered to cope with any challenge.
- See our Autism Spectrum Disorders (ASD) page for more details on Autism.
* Note that these dosage recommendations apply only to Saisei Mirai second-generation GcMAF. GcMAF Therapy If you wish to undergo a GcMAF therapy, contact us by email with details of your illness, current treatment and the quantities of GcMAF you require. Details of prices and payment are given below. High-Dose GcMAF 2.5 ml multi-dose vials (1500 ng/0.5 ml): 2 vials x 2.5 ml GcMAF (1500 ng/0.5 ml) 8 doses 4 vials x 2.5 ml GcMAF (1500 ng/0.5 ml) 16 doses 6 vials x 2.5 ml GcMAF (1500 ng/0.5 ml) 24 doses Keep vials refrigerated at around 2-8 °C for multi-dose use. For long term storage of over one year, vials maybe be kept frozen and defrosted once for multi-dose use. Due to the much improved stability of second-generation GcMAF, freezing is not usually necessary. Please contact us if you require other quantities. GcMAF is a natural immunotherapy product. Variation in GcMAF concentration is due to the normal variation between blood samples. Just as lymphocytes or natural killer cells vary in number between patients and at any given time, so does the amount of Gc-MAF required. We ship Gc-MAF in liquid form in high-quality multi-dose vials. After arrival, vials should be stored in a refrigerator to maintain maximum activity until injection. Gc-MAF is made in our state-of-the-art sterile cell processing facility using a new and improved second-generation method which contains 10-20 times more GcMAF and is more active and stable than other GcMAF products currently available. To ensure the highest level of quality and safety, our GcMAF is carefully tested and screened. All GcMAF products are sterile, filtered with a 0.22 micron filtration system and tested for endotoxins before leaving the laboratory. Our screening process includes the following:
- TPHA test (Syphilis)
- Hepatitis B surface antigen(HBsAg)
- Hepatitis B core antibody (HBcAb)
- Hepatitis B e antigen (HBeAg)
- Hepatitis C virus (HCV) antibody
- HIV antigen and antibody
- Human T-cell lymphotropic virus (HTLV1) antibody
- Endotoxins
GcMAF activity tests Activity experiments conducted by researchers at the University of Tokushima. Note that the test results apply only to 2nd generation GcMAF. GcMAF activity levels under a variety of conditions
- At room temperature (10-15 °C) for 14 days – no significant change in activity
- At 40 °C for 7 days – no significant change in activity
- After 1 year of refrigeration – no significant changes in activity
Summary
- Tests indicate that our second-generation GcMAF is highly temperature-stable and retains its maximum effectiveness even after 1-2 weeks at temperatures as high as 40 °C.
- This makes our Gc-MAF stable enough for shipping worldwide without significant losses in effectiveness during shipment.
- Maximum effectiveness is also retained after one year of refrigerated storage.
IMPORTANT
GcMAF immunotherapy protocol for Cancer
1, The best results will be obtained from 2nd Generation GcMAF injection in combination with Oral Colostrum GcMAF (capsules, spray or candy).
2, In general, GcMAF injection is conducted by subcutaneous administration or intramuscular administration, near the tumours, if possible.
3, Dosage of 2nd Generation GcMAF
1, For cancer prevention
0.5ml GcMAF injection, usually once /week
2, For stage 1 cancer
0.5ml GcMAF injection, usually 1-2 times /week
3, For stage 2 cancer
0.5ml GcMAF injection, usually 1-2 times /week
4, For stage 3 cancer
0.5ml GcMAF injection, usually 2-4 times /week or
1.0ml GcMAF injection, usually 1-3 times /week
5, For stage 4 cancer
0.5ml GcMAF injection, usually every day or
1.0ml GcMAF injection, usually 3-5 /week or
2.5ml GcMAF injection, usually 2-3 times /week
3, Oral colostrum GcMAF is effective for patients with low nutrition and cancer cachexia by improving appetite, increasing energy and decreasing fatigue.
4, Oral colostrum GcMAF is usually taken by oral administration. For example, 20 minutes before breakfast in the morning and before bed time
5, Dosage of Oral Colostrum GcMAF
1, For Adult
4capsules /day, 2 capsules in the morning 20 minutes before breakfast and 2capsules before bed time
2, For child ( 20 kg)
2capsules /day, 1 capsule in the morning 20 minutes before breakfast and 1 capsule before bed time
3, For cancer cachexia
6-8capsules /day, 2 capsules 3-4 times before meal
Second Generation GcMAF should be stored refrigerated for multi-dose use and will stay fully active for over 1 year. However, for expected storage longer than 2 months, vials may be immediately stored frozen and then each vial refrigerated at the beginning of multi-dose use.
Stability tests indicate that 2nd Generation GcMAF is very temperature stable and retains maximum activity even after 4 weeks at room temperature and 1 week at 40 °C (104 °F). Shipping will not affect the activity of the product. Please refer to our Stability of GcMAF experiment report on our website for laboratory data.
Also we recommend to combine other therapies like Tumor Treating Field (TTF) and/or High Dose Vitamin C infusion along with GcMAF for the better results.
https://www.saisei-mirai.or.jp/gan/ttf_tumor_treating_fields_eng.html















