Hyper T/NK Cell Therapy
Treatment of Cancer by Immunotherapy
Background
In a previous study, tumor-infiltrating lymphocytes (TIL) were reported to be effective for advanced cancer patients in experimental and clinical research[1][2] TILs recognize specific antigens expressed by autologous tumor cells. However, the specific antigenicity is too low to achieve a high degree of antigenicity for therapeutic use. In order to solve this problem, Sekine et al. developed a feasible method to obtain large numbers of activated or effective Tlymphocytes.[3][4] Cultivation of peripheral blood lymphocytes with immobilized anti-CD3 monoclonal antibody and human recombinant interleukin-2 induces a rapid and massive proliferation of Tlymphocytes and greatly augments their cytotoxic activity. Administration of these activated TILs demonstrates significant clinical activity in some patients with several types of cancer, making it a useful adoptive immunotherapy.
On the basis of these techniques, we developed the improved cultivation with autologous plasma of patients containing specific antibody for membrane antigens of natural killer (NK) cells. Briefly, peripheral blood lymphocytes are cultivated with immobilized anti-CD3 monoclonal antibody and human recombinant interleukin-2 following Sekine’s method, adding several matrix proteins such as fibronectin in plasma or immobilized monoclonal antibodies such as CD161 for NK cells.[5] This improved cultivation method is used not only to obtain the above-described activated Tlymphocytes but also Hyper T cells and NK cells. Hyper T cells, the name coined by us, are unique and immature multipotent T cells with various capabilities.
These cells decrease in number with advancing age. In particular, a significant decline in number is seen in age groups over 50. Hyper T cells have more widely specific antigenicity for antigens expressed by autologous tumor cells, high ability of proliferation and keep their activity for long periods in vivo.[6] Thus, expansion of Hyper T cells promises to be a suitable and important factor of adoptive immunotherapy against cancer. NK cells represent a unique subset of lymphocytes, distinct from Tlymphocytes. They contribute essential immune systems to host anti-microbial and anti-tumor immunity against cancer or viral infection without the requirement for prior immune sensitization of the host.[7] NK cells function as promising effectors for adoptive immunotherapy against cancer.
In the developed method for the cultivation of Tlymphocytes, Hyper T cells, and NK cells, three cells are cultured simultaneously. Culturing each cell separately has been established by several methods. However, those methods of cultivation have many problems with respect to cost, complexity and safety, and use materials of animal or allogeneic human origin, such as human serum albumin. Therefore, it has been completely adopted in general medical treatment in spite of greatly effective possibility. Due to these reasons, we developed a simple method of culturing these cells, in this case Tlymphocytes, Hyper T cells, and NK cells simultaneously. Using only non-animal and non-human materials for cultivation is important for an immunotherapy designed for cancer patients. Each cell is shown to be effective in medical treatment against cancer, but many problems are also ignored. For example, it is thought to be difficult for NK cells to permeate the tumor tissue due to the size. It is necessary for NK cells to attack the tumor tissue after reducing its size by Tlymphocyte and Hyper Tlymphocyte action. Such a strategy makes it more effective and a sensible immune therapy. The combination of these three immune cells act synergistically to overcome the weakness of each cell type individually.
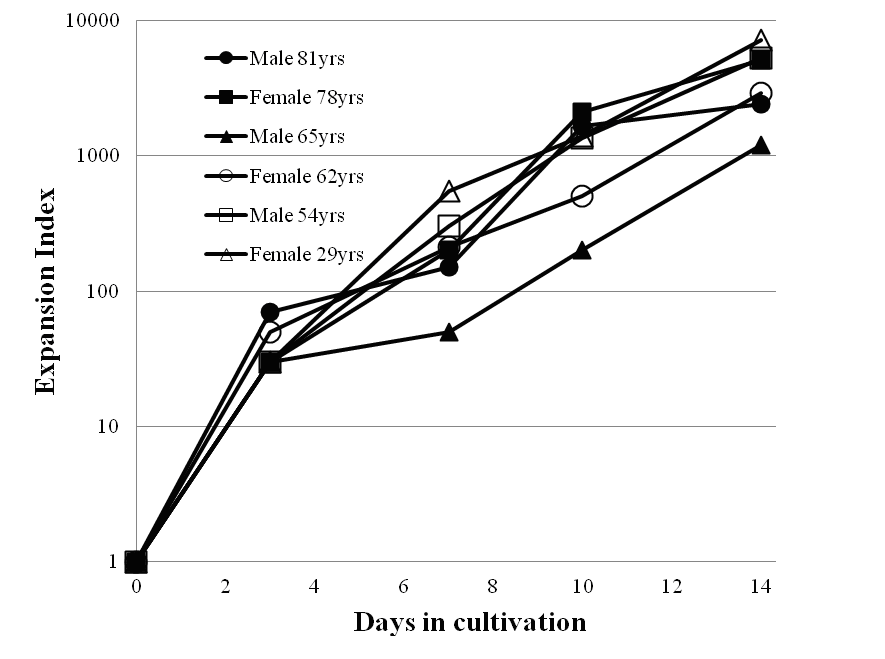
The combination therapy with systemically administered Tlymphocytes, Hyper T cells, and NK cells demonstrated significant clinical activity in some patients with cancer in clinical trials involving more than 100 cases. The mean expansion index of harvested Tlymphocytes, Hyper T cells, and NK cells was about 2500 fold during a cultivation period for 14 days as shown in the figure. Side effects associated with this therapy were only observed very rarely. This method can be of benefit to patients and is a promising immunotherapy. We expect that the described immunotherapy will play a central role in future therapies against certain human cancers, both alone and in combination with other therapies such as GcMAF or hyperthermia treatment.
Growth Behavior of Peripheral Blood Lymphocytes for Representative Patients With Cancer
Peripheral blood lymphocytes were cultivated in the flasks treated with anti-CD3 or anti-CD161 monoclonal antibody and plasma of patient origin for 7 days in the basal medium including human recombinant interleukin-2, followed by 14 days in an untreated culture bag. Cell numbers were counted for 3, 7, 10, and 14 days.
References
- [1] Rosenberg, S.A. Et Al. A New Approach to the Adoptive Immunotherapy of Cancer with Tumor-Infiltrating Lymphocytes. Science 233, 1318-1321 (1986).
- [2] Rosenberg, S.A. Et Al. A Progress Report on the Treatment of 157 Patients With Advanced Cancer Using Lymphokine-Activated Killer Cells And Interleukin-2 or High-Dose Interleukin-2 Alone. N. Eng. J. Med. 316, 889-897 (1987).
- [3] Yamazaki, T. et al. Characterization of Immobilized Anti-CD3 Antibody Activated T Lymphocytes for Use in Adoptive Immunotherapy of Patients with Brain Tumors. NEUROL Med. Chir. (Tokyo) 32, 255-261 (1992).
- [4] Sekine, T. et al. A Feasible Method for Expansion of Peripheral Blood Lymphocytes by Culture with Immobilized Anti-CD3 Monoclonal Antibody and Interleukin-2 For Use in Adoptive Immunotherapy of Cancer Patients. Biomed. And Pharamocother. 47, 73-78 (1993).
- [5] Pozo, D. Et Al. CD161 (Human NKR-P1A) Signaling in NK Cells Involves the Activation of Acid Spingomyelinase. J. Immunol. 176, 2397-2406 (2006).
- [6] Utsuyama, M. Et Al. Differential Age-Change in the Number of CD4+CD45RA+ And CD4+CD29+ T Cell Subsets in Human Peripheral Blood. Mech. Ageing. Dev. 63, 57-68 (1992).
- [7] Ljunggren, H.G. et al. Prospects for the Use of NK Cells in the immunotherapy of Human Cancer. Immunology 7, 329-339 (2007).
Treatment process
Hyper T/NK cell therapy:
- One course (of 6 treatments) is recommended.
- Minimum 3 treatments.
- Administration once every 1-2 weeks.
- After the initial blood sample is taken, it takes 2 weeks for cultivation of the Hyper T/NK cells in our Cell Processing Centre (CPC) in Japan.
- The treatment schedule may vary depending on the patient and circumstances.
Hyper T/NK cells must be kept under special controlled conditions so this therapy is only available to patients who visit our Japanese clinics in Osaka, Kobe and Tokyo.